Flagship industry-sponsored project to design a miniaturized LVDT-based sensing system for spine surgery, enabling real-time depth feedback with ±0.5 mm tolerance.
Project Manager & Mechanical Design Lead & FMEA Analyst
As Project Manager and Mechanical Design Lead, I led a multidisciplinary team to design, prototype, and validate a linear variable differential transformer (LVDT) sensing system integrated into a robotic surgical tool guide. The project aimed to enable surgeons to measure tool insertion depth with ±0.5 mm accuracy in real time.
My role involved leading design reviews, managing documentation, coordinating between mechanical and electrical subteams, and building the physical calibration and testing rig used to validate the sensor system. I also conducted the FMEA analysis to identify risks across the mechanical assembly, electrical interfacing, and data acquisition processes.
Prototype
I fabricated the complete test rig independently, assembling the mechanical and electrical components for validation.
The final prototype included:
Miniaturized LVDT Sensor: Custom coil configuration (80-turn secondary coils, 160-turn primary).
Signal Conditioning Circuit: CN0301 board and SDP controller for amplitude demodulation and signal conversion.
Custom Aluminum Housing: Machined for sterilization compliance and to reduce electromagnetic coupling.
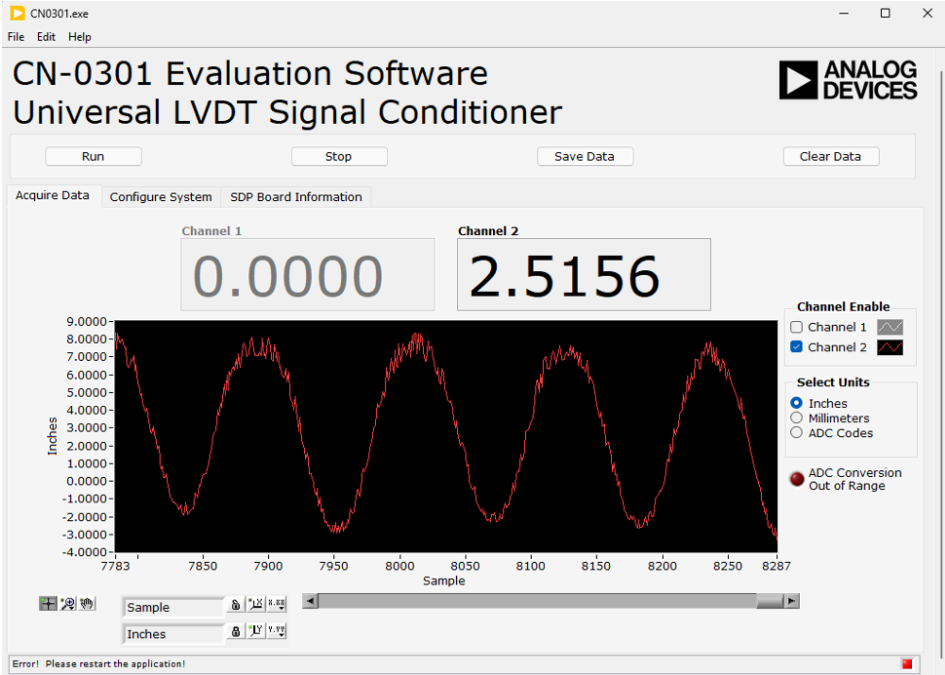
Integration & Visualization: Sensor outputs processed in LabVIEW and MATLAB, enabling live displacement readings and calibration analysis.
This setup allowed us to correlate sensor output voltage to physical displacement, producing a highly linear calibration curve.
Results
Through iterative calibration, the sensor achieved a measurement tolerance of ±0.5 mm over a 20 mm range, meeting and exceeding Stryker’s design goals. The precision rectifier and filter circuits reduced noise significantly, resulting in a stable DC output that accurately tracked core movement. The LabVIEW interface displayed real-time displacement data, allowing visual verification of sensor response.
Problem
In robotic-assisted spine surgeries, surgeons rely on mechanical hard stops for tool positioning, which do not provide continuous positional feedback during the procedure. This lack of feedback increases the potential for over-penetration and surgical error.
Our goal was to design a compact, sterilizable sensing system that could continuously measure displacement through an inductive LVDT sensor, translating micro-level core movements into a precise DC output. The system needed to integrate seamlessly into Stryker’s existing robotic workflow, meet strict accuracy requirements, and be compatible with a sterilizable tool guide housing.
Process
Project Management & Collaboration
As project manager, I established the weekly timeline, delegated sub-tasks, and ensured that mechanical, electrical, and software teams progressed cohesively. I maintained consistent communication with Stryker engineers through biweekly design review meetings, ensuring deliverables aligned with industry requirements.
I also created and maintained comprehensive documentation, including meeting notes, design iterations, test procedures, and the FMEA report, which helped the team meet every gate deadline ahead of schedule.
Mechanical Design & Test Rig Development
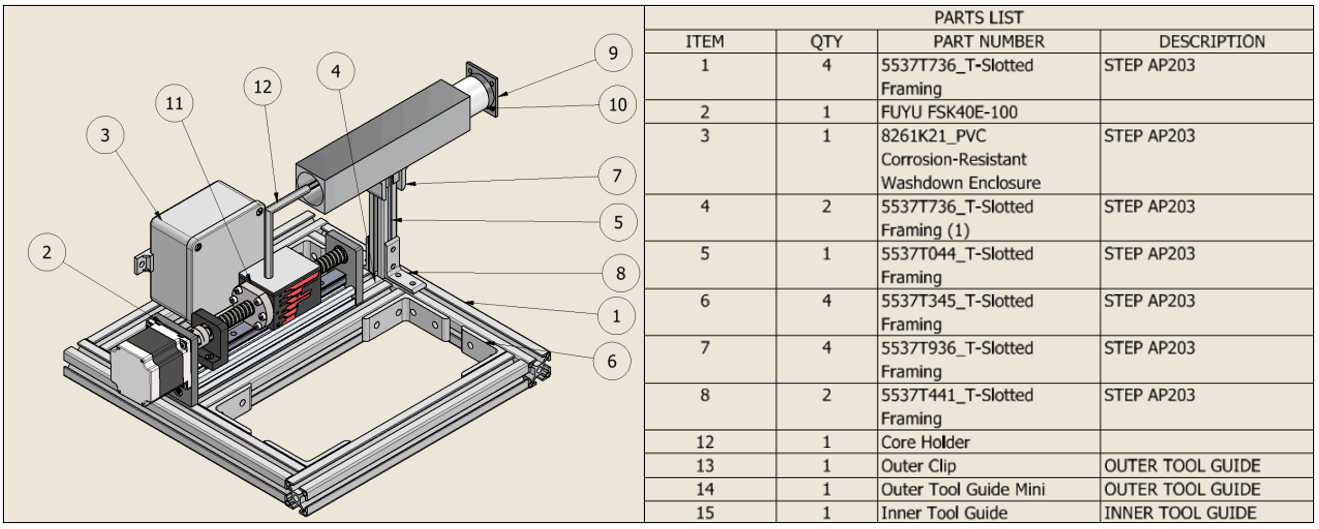
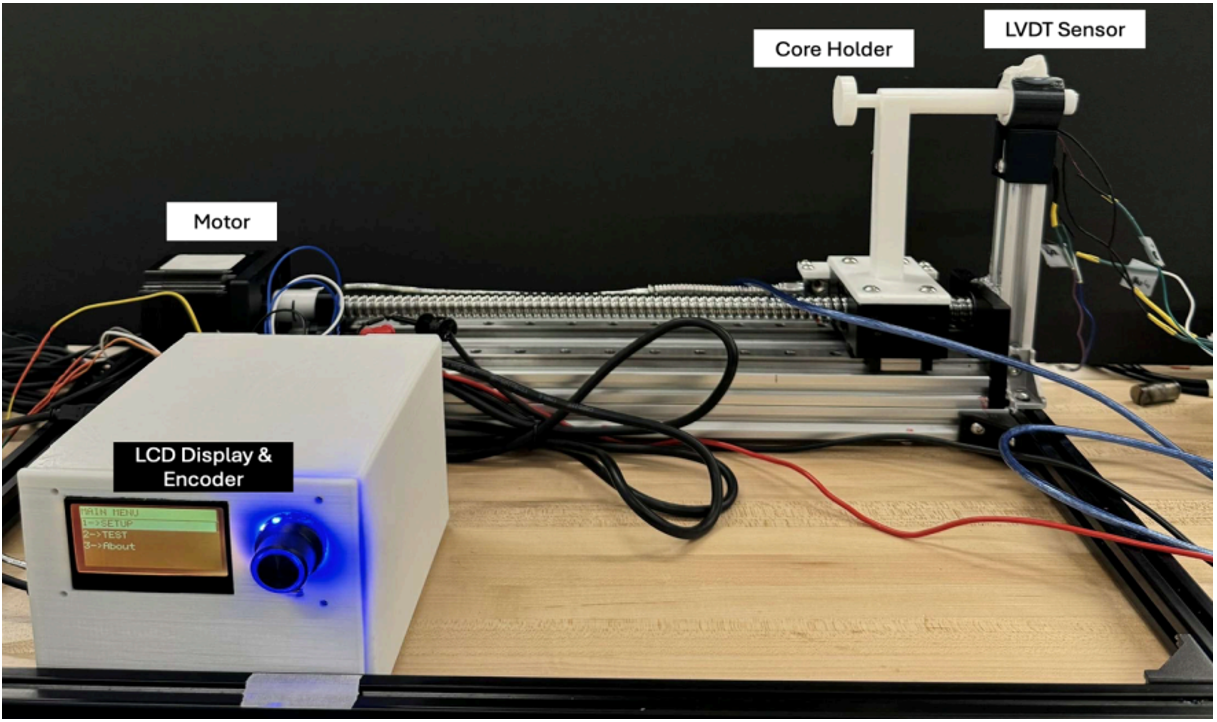
As the Mechanical Design Lead, I designed the calibration and testing rig used to evaluate sensor accuracy and repeatability.
The rig was constructed from 80/20 aluminum rails, providing a modular structure for precise linear movement.
It incorporated a Digital Read-Out (DRO) displacement sensor, a NEMA 23 stepper motor, and a linear guide assembly, enabling movement of the LVDT core in 1 mm increments.
The setup was calibrated to ensure alignment between mechanical displacement and sensor response.
This test rig became the foundation for all sensor validation experiments. I personally fabricated, assembled, and wired the rig components, confirming functionality and data repeatability.
FMEA and Risk Mitigation
I performed a Failure Modes and Effects Analysis (FMEA) on key subsystems including the LVDT coil winding, DRO sensor, and data acquisition circuitry. Major risks identified included:
Noise interference from surrounding electronics,
Mechanical misalignment of the test rig, and
Coil damage or signal loss due to solder joint stress.
Mitigation steps included foil shielding for EMI protection, heat-shrink insulation, and precision assembly jigs to improve reliability.
Impact & Takeaways
This project resulted in a fully validated, sterilization-compliant prototype ready for further system integration within Stryker’s robotic surgery division. As project manager, I learned to balance leadership and technical execution, ensuring the team remained cohesive and productive across multiple subsystems.
The experience deepened my understanding of sensor design, calibration, EMI mitigation, and medical device standards, while reinforcing the importance of documentation, testing, and precision mechanical design. Ultimately, this project demonstrated how thoughtful engineering can directly enhance surgical safety and patient outcomes.